
A 77 year old male with past medical history of HTN, HLD, prediabetes, obesity, COPD, AAA who initially underwent an CT abdomen pelvis WWO in 6/2013 for evaluation for nephrolithiasis which noted a 1.3 x 2.2 cm cystic structure in the neck of pancreas. Subsequently, underwent EGD with EUS in 2013 which confirmed a 2.1 cm x 1.8 cm pancreatic neck cyst s/p fine needle aspiration (FNA) with fluid Carcinoembryonic Antigen (CEA) levels of 415 ng/mL with fluid amylase of 1,948 U/L. Surveillance EGD with EUS in 3/2016 noted 1.9 mm x 1.8 mm anechoic cyst in pancreatic neck s/p FNA with fluid CEA to be 419 ng/mL with fluid amylase of 16,398 U/L. A CT A/P with IV contrast in 1/2023 performed at an ER visit for abdominal pain noted pancreatic neck cyst now measuring 3.1 cm with minimal pancreatic duct dilation.
The patient was subsequently lost to follow up until 2025 when he presented to the GI clinic for pancreatic cyst evaluation. An MRI/MRCP performed 3/2025 revealed the cyst had now enlarged to 4.0 cm x 1.8 cm x 4.3 cm with mild dilation of the pancreatic duct in the proximal body up to 6 mm (Figure 1).
He was then referred for a repeat endoscopic ultrasound in 3/2025 which identified a thick-walled measuring 4.0 cm by 1.8 cm in maximal dimension located the pancreatic neck with connection to the main pancreatic duct. The main pancreatic duct was also dilated to 7 mm with internal hyperechoic debris. There was no endosonographic evidence of mural nodule, eccentric wall thickening or mass lesions. FNA cytology was negative for malignancy however showed papillary fragments of mucinous epithelium, suggestive of a low-grade dysplasia. There was no evidence of high-grade dysplasia or invasion. CA 19-9 was 13 U/mL at the time.
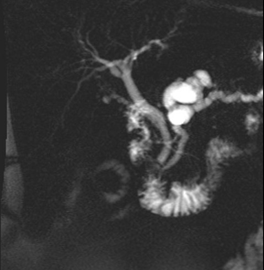
Figure 1. (A) MRI/MRCP showing 4.0 x 1.8 x 4.3 cm cystic lesion within the neck of the pancreas.
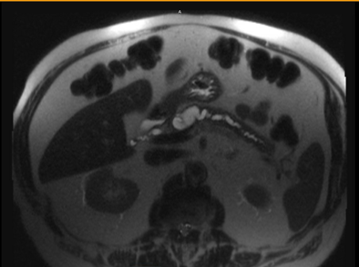
(B) which communicates with the main pancreatic duct which is dilated measuring 6 mm. There are no solid or enhancing components. Images from the library of Rush University.
Question
Based on the imaging and fluid analysis, which type of pancreatic cystic neoplasm (PCN) would this be considered?
A) Serous Cystadenoma/Serous Cystic Neoplasm (SCN)
B) Pseudocyst
C) Intraductal Papillary Mucinous Neoplasm (IPMN)
D) Mucinous Cystic Neoplasm (MCN)
E) Pancreatic Ductal Adenocarcinoma (PDAC)
The correct answer is C, Intraductal papillary mucinous neoplasm (IPMN)
Intervention
Given the patient had a suspected mixed-type IPMN with high risk features he was referred to surgery for surgical consultation for discussion of a pancreaticoduodenectomy. After discussion, he elected not to pursue surgery given the risks. His case was then presented at our multidisciplinary pancreatobiliary conference. Given the features of his pancreatic cyst, the decision was made to proceed with EUS-guided chemoablation. He subsequently underwent an EUS-guided chemoablation of his pancreatic cyst using Paclitaxel and tolerated the procedure well without complications. He is now undergoing active surveillance with subsequent EUS and MRI.
Practice Pearls
Epidemiology
Given the accessibility to quality cross sectional imaging in our era, there has been an increase in the incidence of pancreatic cystic neoplasms (PCNs) in our patient population. The incidence of incidental cysts has a rate between 2 to 15% of patients undergoing computed tomography (CT) and magnetic resonance imaging (MRI).1 This finding combined with the poor prognosis of pancreatic cancer leads to significant concern for providers and patients. Furthermore, with an estimate of 20% of pancreatic cancers are thought to arise from the progression of mucinous-type pancreatic cysts, a thorough understanding of their diagnosis and treatment is needed to improve patient outcomes.2
Pancreatic cysts can initially be subdivided into mucinous or non-mucinous lesions depending on the presence of columnar epithelium which produces mucous and has malignant potential.3 Non-mucinous cysts include serous cystadenomas, pseudocysts, lymphoepithelial cysts, and simple cysts and are typically considered to carry no or negligible risk for malignant conversion.3,4 Mucinous cysts include intraductal papillary mucinous neoplasms (IPMNs), mucinous cystic neoplasm (MCN).4 However, providers also need to be aware of additional cysts that frequently present with mixed solid and cystic components including cystic neuroendocrine tumors, solid pseudopapillary neoplasms, and pancreatic adenocarcinomas with cystic degeneration.1
IPMNs were first described in 1982 and were considered a rare disease up until the early 2000.5 They are considered the most prevalent pancreatic cystic lesions and constitute about 80% of incidentally diagnosed PCNs .10 Three major categories of IPMNs exist: main duct (MD) -IPMNs, branch duct (BD) - IPMNs, mixed-type (M)- IPMNs (have features of both MD and BD).3,4 As mucinous cysts such as IPMNs have premalignant potential, active surveillance and therapeutic management strategies for cysts with high-risk features are required.
Pathogenesis/Pathology
IPMNs originate from the epithelial cells of the pancreatic ducts.4 Their main characteristic is cell proliferation in the form of mucin-producing goblet cells interspersed with dysplastic papillary projections that leads to cystic dilatation.4 IPMNs can occur in any part of the pancreas, with the head being the most common location (70%) followed by body/tail (20%) and multifocal occurrences (5-10%).5 A MD-IPMN results in dilation of the main pancreatic duct > 5 mm without other identifiable causes while the BD-IPMNs communicate with the main pancreatic duct via a dilated side branch but do not cause main duct dilation. The risk of IPMN progression to malignancy is dependent on several features of the cysts including the type, size and the presence of “high-risk” features with MD-IPMNs carrying the highest risk for malignant conversion.3,4
Diagnosis
Currently the clinical management of IPMNs relies on several guidelines based on laboratory, endoscopic, cytologic, and imaging findings to appropriately select the best candidates for surgery.3,5,6 The initial evaluation for newly diagnosed IPMNs is dedicated imaging with contrast-enhanced MRI of the pancreas with magnetic resonance cholangiopancreatography (MRCP) or a pancreas protocol CT scan or endoscopic ultrasound (EUS) in patients unable to undergo MRI.3,5, 6 “High-risk” or worrisome features seen on clinical presentation, imaging or endosonographic evaluation include:1,4,6
Clinical Presentation:
- New onset diabetes
- New onset obstructive jaundice
- Acute pancreatitis attributed to the cyst
Imaging and Endosonographic Characteristics
- Dilated main pancreatic duct > 10 mm (some guidelines reporting > 5 mm)
- Cyst diameter >3 cm
- Thickened (or enhancing) cyst wall
- Mural nodule >5 mm
- Interval cyst growth > 3-5 mm/year
- Solid component (mass)
- Abrupt cut off of the main pancreatic duct
Laboratory and Pathology/Cytology Characteristics
- Elevated CA19-9 level
- Positive or worrisome cytology on cyst fluid aspiration
EUS with fine needle aspiration of the cyst can be performed to obtain cyst fluid (typically in cysts > 1.5 to 2 cm in size) for biochemical analyses to differentiate the type of cyst (i.e. facilitate the diagnosis of mucinous vs non-mucinous etiology). Aspirated cyst fluid is sent for testing that includes CEA level, amylase, glucose concentration, DNA mutational markers, and cytology to evaluate for dysplasia (Table 1). Elevated fluid amylase indicates connection with the pancreatic duct and is typically elevated in IPMNs. Fluid CEA may add in determining mucinous etiology but does not help distinguishing malignancy in mucinous PCNs.
| Pancreatic cyst | Fluid CEA | Fluid Amylase | Imaging Characteristic | Malignant Potential |
|---|
| Pseudocyst | Low | High | Well circumscribed, oval or round, anechoic on EUS, low attenuation on CT | None |
| SCN | Low | Low | Microcystic/honeycomb; 30% central scar | None |
| IPMN | High | High | Dilated pd | Yes |
| MCN | High | Low | Multiple loculated or septated, some with peripheral calcifications, mostly in tail | < 3 cm: < 5%, >3 cm: high |
| pNET | Low | Low | Well circumscribed cystic lesion with peripheral rim enhancement | Yes (minimal risk) |
Table adapted from Ngamruengphong et al. and Ohtsuka et. al.6,7; Fluid CEA (<192 ng/ml) = low
Management
- Surgical resection
- Theoretically, the resection of pre-malignant pancreatic cystic lesions is the best strategy for preventing malignant progression to pancreatic adenocarcinoma6
- High-risk BD-IPMN should undergo partial pancreatectomy with radical pancreatectomy with LN dissection reserved for cases when invasive carcinoma is suspected.3
- Careful consideration - Mortality rates from surgical resection for pancreatic cysts is estimated to be between 1 – 7% and morbidity is as high as 64% (avg 30%)4, 7
- EUS-guided chemoablation
- A novel, minimally invasive treatment modality for premalignant pancreatic cystic lesions that is safe, cost-effective option for long term control for premalignant pancreatic cysts.2,9
- The chemo agent typically used for this procedure is Paclitaxel and gemcitabine.2,9
- EUS-FNI of paclitaxel-based regimens resulted in a complete resolution of 63.6% of mucinous PCNs with an adverse event rate of 15%10
- Long-term results showing an oncological benefit are lacking.6
- Surveillance
- Contrast enhanced MRI with MRCP is considered preferred follow up method in cases for IPMNs since it shows greater sensitivity compared to CT in identifying communication with the pancreatic ducts and presence of high-risk stigmata and worrisome features 1,6
- Current guidelines are slightly variable on the recommended surveillance timelines of low risk BD-IPMN but are generally stratified by largest cyst size:1
- BD-IPMN <10 mm : MRI at 6 months to 1 year, then every 2 years.
- BD_IPMN 10 mm to 20 mm: MRI at 6 months to 1 year, then annually for 2-5 years. Then if stable, can extend to every 2 years.
- BD-IPMN > 20 mm and < 30 mm : MRI/EUS every 6 months for 2-3 years, then annually, if stable.
- BD-IPMN > 30 mm: MRI alternating with EUS every 3-6 months.
- Long term surveillance of small unchanged BD-IPMN is controversial. Some guidelines recommend stopping surveillance if stable for 5 years,11 while others suggest discontinuing when the patient is determined to no longer be a surgical candidate.1
References
- Gardner TB, Park WG, Allen PJ. Diagnosis and Management of Pancreatic Cysts. Gastroenterology. 2024 Aug;167(3):454-468. doi: 10.1053/j.gastro.2024.02.041. Epub 2024 Mar 3. PMID: 38442782.
- Moyer MT, Maranki JL, DeWitt JM. EUS-Guided Pancreatic Cyst Ablation: a Clinical and Technical Review. Curr Gastroenterol Rep. 2019 Apr 23;21(5):19. doi: 10.1007/s11894-019-0686-5. PMID: 31016391; PMCID: PMC11613143.
- Ayoub F, Davis AM, Chapman CG. Pancreatic Cysts-An Overview and Summary of Society Guidelines, 2021. JAMA. 2021 Jan 26;325(4):391-392. doi: 10.1001/jama.2020.18678. PMID: 33496762.
- Buerlein RCD, Shami VM. Management of pancreatic cysts and guidelines: what the gastroenterologist needs to know. Ther Adv Gastrointest Endosc. 2021 Sep 23;14:26317745211045769. doi: 10.1177/26317745211045769. PMID: 34589706; PMCID: PMC8474323.
- Moris D, Liapis I, Gupta P, Ziogas IA, Karachaliou GS, Dimitrokallis N, Nguyen B, Radkani P. An Overview for Clinicians on Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas. Cancers (Basel). 2024 Nov 14;16(22):3825. doi: 10.3390/cancers16223825. PMID: 39594780; PMCID: PMC11593033.
- Ohtsuka T, Fernandez-Del Castillo C, Furukawa T, Hijioka S, Jang JY, Lennon AM, Miyasaka Y, Ohno E, Salvia R, Wolfgang CL, Wood LD. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024 Mar;24(2):255-270. doi: 10.1016/j.pan.2023.12.009. Epub 2023 Dec 28. PMID: 38182527.
- Ngamruengphong S, Lennon AM. Analysis of Pancreatic Cyst Fluid. Surg Pathol Clin. 2016 Dec;9(4):677-684. doi: 10.1016/j.path.2016.05.010. PMID: 27926366; PMCID: PMC5145907.
- Yadav RK, Jiang X, Chen J. Differentiating benign from malignant pancreatic cysts on computed tomography. Eur J Radiol Open. 2020 Nov 1;7:100278. doi: 10.1016/j.ejro.2020.100278. PMID: 33163586; PMCID: PMC7607418.
- Koehler B, Ryoo DY, Krishna SG. A Review of Endoscopic Ultrasound-Guided Chemoablative Techniques for Pancreatic Cystic Lesions. Diagnostics (Basel). 2023 Jan 17;13(3):344. doi: 10.3390/diagnostics13030344. PMID: 36766449; PMCID: PMC9914819.
- Krishna SG, Ardeshna DR, Shah ZK, Hart PA, Culp S, Jones D, Chen W, Papachristou GI, Han S, Lee PJ, Shah H, Pawlik TM, Dillhoff M, Manilchuk A, Cloyd J JM, Ejaz A, Fry M, Noonan AM. Intracystic injection of large surface area microparticle paclitaxel for chemoablation of intraductal papillary mucinous neoplasms: Insights from an expanded access protocol. Pancreatology. 2024 Mar;24(2):289-297. doi: 10.1016/j.pan.2023.12.014. Epub 2024 Jan 11. PMID: 38238194.
- Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015 Apr;148(4):819-22; quize12-3. doi: 10.1053/j.gastro.2015.01.015. PMID: 25805375.

Sarah Kosinski DNP, APRN, FNP-BC, is the Lead Advanced Practice Provider for the Digestive Diseases and Nutrition Division at Rush University Medical Center with 7 years of experience in gastroenterology. She received her doctorate in Nursing Practice from Rush University In 2018. She is currently serving on the ASGE APP Committee and is the adjunct faculty for the College of Nursing at Rush University.

Christopher Chapman, MD, is a gastroenterologist with an expertise in interventional endoscopy. Dr. Chapman is a member of the Center for Interventional and Therapeutic Endoscopy (CITE) at Rush University Medical Center in Chicago, IL. Dr. Chapman has a special interest in endoscopic bariatric therapies, such as intragastric balloon placement, and endoscopic suturing (endoscopic sleeve gastroplasty and bariatric surgery revision). His extensive research has been published in multiple peer-reviewed journals, including Gastroenterology, Gastrointestinal Endoscopy, Clinical Endoscopy and Nature Scientific Reports. He currently serves as chair of the ASGE Medical Simulation and Education Technology Committee.